Webinar Series | Spring 2022

As very recent history has illustrated vividly, societies face challenges and threats from emerging and mutating viruses. This in turn highlights the importance of techniques which can be used to gain insights into their morphology and interactions both in vivo and in vitro. Indeed, understanding their delicate capsid architecture, stability in various environments, detailed mechanism of infecting cells and spread between humans are few of many vital questions scientists must answer in order to develop techniques to better treat and contain viral infections in the future.
But how can we see viruses? The two most common techniques are transition electron microscopy (TEM and Cryo-TEM) and X-ray diffraction analysis. Both techniques, especially Cryo-TEM, could potentially reveal detail near the atomic level of resolution. Yet, both techniques require vacuum environments and disruptive sample preparation techniques such as metal staining. This, in principle, could have an impact on the samples and certainly is not compatible with physiological conditions.
Another powerful, yet less frequently used technique, is atomic force microscopy (AFM). It has the distinction of both possessing comparable resolution to TEM or X-ray diffraction analysis (here we demonstrate a resolution of ~1 nm) with several unique advantages. First among these is the ability to look at viruses and other biological objects in real time and in both native and biologically relevant environments, i.e. in vitro and, potentially, in vivo. This enables AFM to be used to observe viruses on live cell surfaces as they infect them. Secondly, the ability to control the environment of the sample (i.e. temperature, solvent or substrate) and to measure additional properties such as mechanical or electrochemical properties using functional AFM modes enables a host of sophisticated experiments to be performed. Together, the advantages offered by AFM enable both fundamental and very practical questions about the management of viral infections to be answered.
In this webinar we will look at some advantages and challenges at looking at viruses with Atomic Force Microscopy both in air and liquid environments.
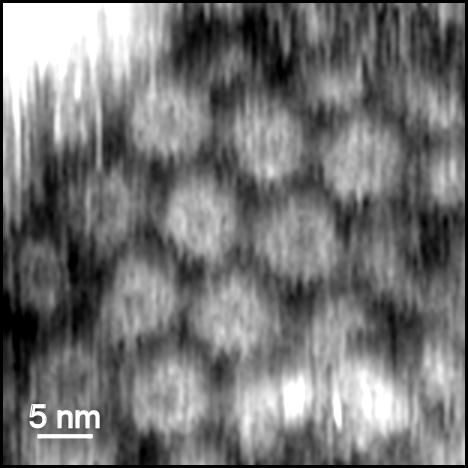
Fig. 1. High resolution AFM image of the surface of an individual virus on mica performed in a liquid environment (water).
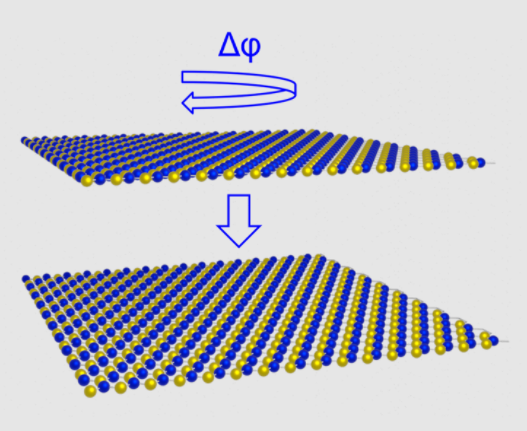
A schematic of the formation of parallel stacked bilayer hBN is shown in addition to a contact potential difference map measured using sideband KPFM.

Image caption

Image caption

Image caption

Vladimir received his PhD in Chemistry from Moscow University in 2008. Then, he moved to the University of Heidelberg and specialized in X-ray photoelectron spectroscopy of thin films, following by the position at the University of Nottingham, where he discovered his passion for Scanning Probe Microscopy (SPM), and became a strong advocate of SPM techniques to unlock structure and properties at nanoscale. He pioneered the use of higher eigenmodes of standard cantilevers to routinely achieve resolution that was previously thought to be exclusively limited to STM and UHV-STM. Vladimir published more than 40 scientific papers, including three in Nature family journals. He left academia in 2018 to contribute to the industrial site of SPM technology.
